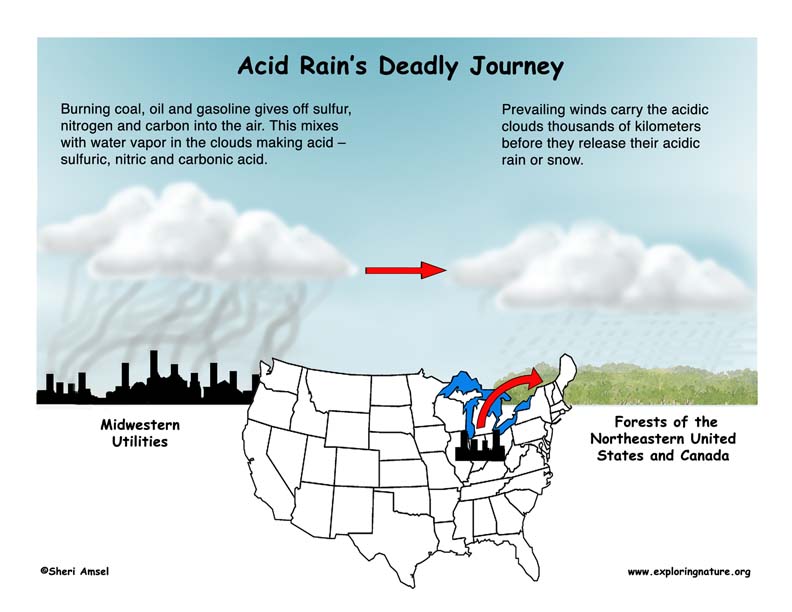
Burning coal, oil and gasoline gives off sulfur, nitrogen and carbon into the air. These emissions combine with water vapor in the clouds and make acid – sulfuric, nitric and carbonic acid. These acidic clouds are carried by the wind, sometimes thousands of kilometers. The wind patterns of North America carry pollutants from busy cities, factories and industrial centers in the Midwest and drops them on the Northeastern U.S. and Canada. They fall to Earth as acid rain or snow.
How do we know when something is acidic?
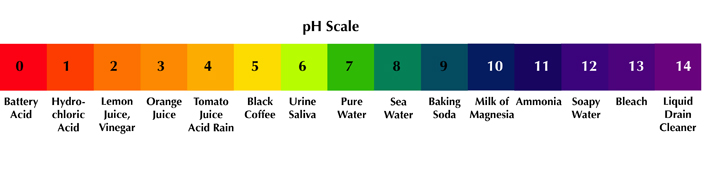
The acidity of a substance is referred to as its pH – which is the measurement of whether it is acidic or alkaline (base). The number scale used to measure pH is from 1 to 14 with 1 being the most acidic and 14 being the most alkaline (base). 7 is considered neutral, neither acid or base. In nature, everything has their own pH.
For example, lemon juice or vinegar have a pH of 2. They are considered to be "acid." While baking soda has a pH of 9 and bleach has a pH of 13. They are considered to be "base" or "basic" or "alkaline". Compounds at either end of the pH scale are considered "reactive" and will burn the skin. Anyone who has had lemon juice squirt in your eye can attest to this.
Each level of the pH scale represents the power of 10. So if the pH of lemon juice is 2 and orange juice is 3, then lemon juice is 10 times more acidic than orange juice. Sulfuric acid measures 1 on the pH scale. It is 10 times more acidic than lemon juice and 100 times more acidic than orange juice (10 x 10).
Different kinds of natural communities can tolerate different levels of acid and base. Normal rain is slightly acidic naturally with a pH of 5.6. This is because the rain combines with carbon dioxide in our air. This makes a very mild form of carbonic acid naturally. The average pH of a healthy freshwater lake is about 6.5. If a lake receives too much acid rain and the pH begins to fall, fish, frogs and other lake animals may begin to die off.
Acid rain levels have decreased because of new regulations, but not all the ecosystems are all recovering. The forests that have been affected by decades of acidity are much more sensitive because their natural buffers have been damaged (the calcium and magnesium in the soil have been leeched out). This is because soils take thousands of years to develop so recovery is very, very slow. Despite the decrease in acid rain, the deciduous forests of New England may still experience a major die off.
Much has been done to decrease acid rain in the last few decades. In 1990, congress passed Clean Air Act Amendments which require companies to reduce their sulfur emissions. One way they do this is by installing "scrubbers" in their smokestacks. They release a liquid alkaline that mixes with the emissions and traps 80-95% of the sulfur pollutants before it can escape into the air.
Many scientists believe that the remaining levels of sulfur oxides coming from smokestacks needs to be decreased further.
Any decrease in carbon, sulfur and nitrogen emissions is helpful. Individuals can help by:
Understanding Acid Rain
1. The pH scale goes from 1 to 14. What number is the most acid?
2. Can you name something in your household that would be considered acid?
3. Can you name something in your household that would be considered base or alkaline?
4. Where does normal rain fall on the pH scale - a little acid or base?
Why?
5. What human actions cause acid rain?
6. Why doesn’t the acid rain fall right where it is made?
7. Name two effects of acid rain.
When you research information you must cite the reference. Citing for websites is different from citing from books, magazines and periodicals. The style of citing shown here is from the MLA Style Citations (Modern Language Association).
When citing a WEBSITE the general format is as follows.
Author Last Name, First Name(s). "Title: Subtitle of Part of Web Page, if appropriate." Title: Subtitle: Section of Page if appropriate. Sponsoring/Publishing Agency, If Given. Additional significant descriptive information. Date of Electronic Publication or other Date, such as Last Updated. Day Month Year of access < URL >.
Amsel, Sheri. "Acid Rain" Exploring Nature Educational Resource ©2005-2024. December 14, 2024
< http://www.exploringnature.org/db/view/Acid-Rain >